Proprietary Technologies
The range of technologies we have accumulated over a history spanning
more than nine decades has proven to be a powerful asset.
This section showcases some of Yamamoto Chemicals' proprietary technologies.
Specialized reactions and precision technologies
Specialized reactions
- Electrophilic substitution reaction
- Halogenation, nitration, sulfonation, Friedel-Crafts, formylation
- Nucleophilic substitution reaction
- Alkoxylation, amination, thioaklylation
- Condensation reaction
- Esterification, amidation, imidation, etherification, heterocyclization
- Addition reaction
- Diels‐Alder reaction
- Reduction reaction
- Hydrogenation reaction, sodium hydrosulfide reduction reaction
- C-C binding reaction
- Ullmann reaction- Suzuki coupling- Sonogashira reaction
- C-N binding reaction
- Diazonium coupling reaction- N-allylation
Purification technologies
Sublimation refining
Freeze drying
Proprietary compounds
-
Phthalocyanine compounds
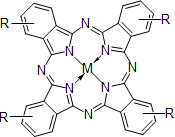
- Naphthalocyanine compounds
- Tetraazaporphyrin compounds
- Anthraquinone compounds
-
Azo compounds (typical structures)
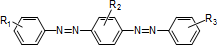
-
Cyanine compounds
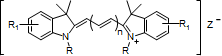
-
Fluoran compounds
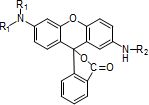
-
Quinophthalone compounds
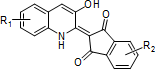
Proprietary reactions and technologies
Reactions using sulfuric acid
| Dehydration-condensation reaction | Triphenylmethane derivative |
|---|---|
| Anthraquinone derivative | |
| Triphenylmethane derivative |
Reactions using aluminum chloride
| Acylation reaction | Benzoyl derivative |
|---|---|
| Benzoyl ketone derivative |
Reactions using acetic anhydride
| Acetylation reaction | N-, C-acetyl derivative |
|---|---|
| Dehydration-condensation reaction | Phthalide derivative |
Reactions using dialkyl sulfite
| Alkylation reaction | N-Methyl derivative |
|---|---|
| N-Ethyl derivative |
Reactions using alkyl halide
| N-Alkylation reaction | N-Alkyl derivative |
|---|---|
| N-Dialkyl derivative | |
| O-Alkylation reaction | Alkoxy derivative |
| Esterification reaction | Ester derivative |
Reactions using aryl halide
| N-Aryl reaction | N-Aryl derivative |
|---|---|
| N-Diaryl derivative | |
| N-Triaryl derivative | |
| O-Aryl reaction | Phenoxy derivative |
Reactions using sodium nitrite
| Diazotization reaction | Azo dye |
|---|---|
| Tetrazotization reaction | Azo dye |
Reactions using chlorosulfuric acid
| Chlorosulfonation reaction | Sulfonyl derivative |
|---|---|
| Sulfonic acid derivative |
Carbon-carbon cross coupling reaction
| Bisaryl derivative |
Heterocyclic forming reaction
| Indole derivative |
| Isoindole derivative |
| Pyrrole derivative |
| Barbituric acid derivative |
| Phenothiazine derivative |
Vilsmeier reaction
| Formylation reaction |
| Cyanation reaction |
Halogenation reaction
| Bromination reaction |
| Chlorination reaction |
Other
| Phthalocyanine derivative |
| Naphthalocyanine derivative |
| Tetraazaporphyrin derivative |
| Cyanine derivative |
| Azo metal complex |
Contact Us
For consultations or queries regarding
Yamamoto Chemicals and its products,
feel free to contact us via telephone
or the email inquiry form.
Telephone queries: [Tokyo Office]
+81-3-6402-2191Weekdays 9:00 a.m. to 5:00 p.m.,
closed Saturdays, Sundays and public holidays